
For this topic, we will have a seri about this material type: Stainless steel material.
1. What is Stainless Steel?
2. Corrosion Mechanisms
3. Environmental Factors That Influence Corrosion in Water
4. Construction and Fabrication Influences on Stainless Steel Corrosion
5. Material Handling After Fabrication and During Installation / Stock Materials and Fabrication of Stainless Steel Parts
6. CORROSION RESISTANCE TESTING METHODS
7. UTILITY AND FABRICATOR EXPERIENCES WITH STAINLESS STEEL CORROSION
8. GUIDELINES FOR STAINLESS STEEL USE
9. Other…
Okay! Now we start. Are you ready?
I. What is Stainless Steel?
A metallic alloy (i.e., metal made of multiple elements such as chromium, nickel, and molybdenum) is generally considered to be “stainless” when its chromium content is greater than about 12 percent by weight ( some document mention it is about 10.5% chromium ), with the balance being iron, higher alloyed stainless steels have higher levels of chromium. Chromium provides corrosion resistance to these alloys by forming a thin, adherent, corrosion-resistant oxide film on a clean (e.g., pickled, wire/rotary brushed, or ground) surface (of the alloy).
When exposed to oxygen, whether in air or even in water, this layer will naturally form and will help to prevent corrosion of stainless steel beneath it. The effectiveness of this protective oxide layer can become compromised when the original oxide surface layer becomes damaged or scratched, but rapidly reforms its protective film in the presence of oxygen.
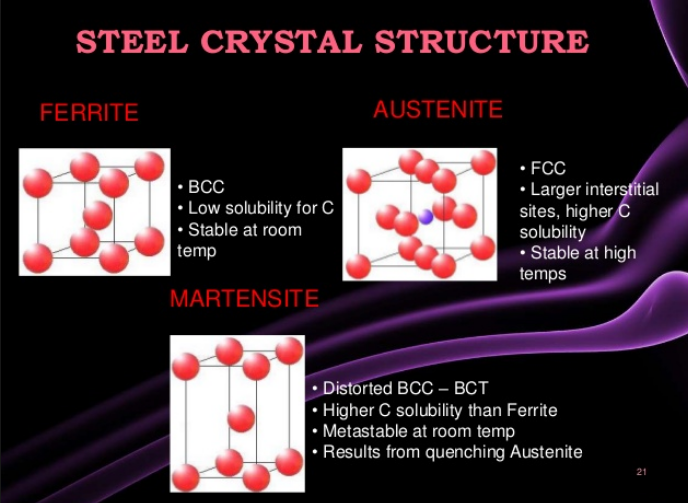
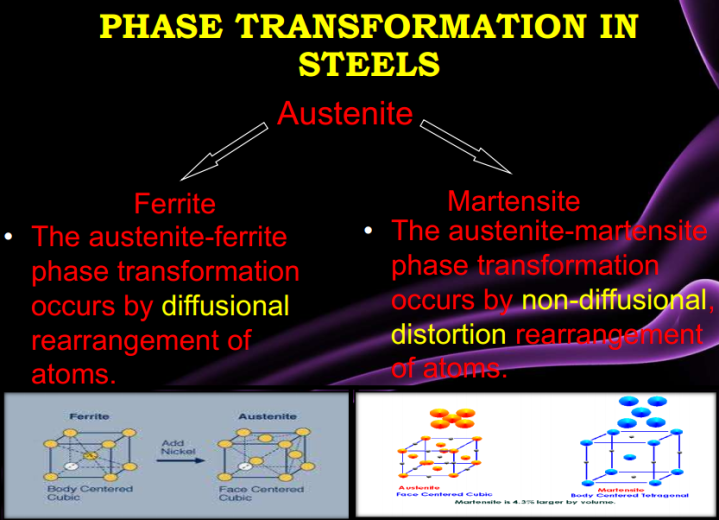
There are five families of stainless steel, each characterized by their structure: martensitic, ferritic, austenitic, duplex (50/50 austenite/ferrite mixed structure), and precipitation-hardened structures. The families are characterized by their microstructures, which result from their particular compositions.
The properties typical to the different structures of stainless steel alloys are as follows:
1 & 2: Ferritic and martensitic alloys are iron-chromium alloys. Martensitic alloys are hardenable by heat treatment, while ferritic alloys are not. Both ferritic and martensiticgrades belong to the 400 series of stainless steels, which provide strength but only minimal corrosion resistance.
* Ferritic & Martensitic Grades:
- Alloyed with Chromium (but have no or low Nickel content)
- Examples – 13% Cr (Ferritic) 13%Cr +4%Ni
- Ferritic grades have ferrite as main phase & so can be magnetised
- Martensitic grades have martensitic as main phase
- Similar characteristics to C & Mn steels but with improved corrosion resistance
- Not suitable for very low temp. but some Ferritic grades used for good resistance to scaling at high temperatures
3. Austenitic stainless steels are iron-chromium-nickel alloys (nickel provides malleability and weldability to this series of alloys). These materials are known as the 300 series of stainless steel alloys, which offer corrosion resistance to a wide variety of waters. L-grade 300 series grades such as 304L have guaranteed low carbon contents, which is important for welding. The L-grades however have slightly lower minimum yield and tensile strengths. Dual grade, such as 304/304L has both the guaranteed low carbon of the L-grade and the guaranteed minimum strength of the non-L grade. The molybdenum-containing 316/316L dual grade is also commonly available. The 300 series alloys have reasonable strength in the annealed condition, and can be made much stronger and harder by cold work, but this is not practical in most water applications. Higher alloyed austenitic stainless steels are recognized as the super-austenitic stainless, e.g., 254SMO, 654SMO, and AL6XN…
* Austenitic Grades:
- Alloyed with Chromium & Nickel
- Examples – 304 & 316 (18%Cr + 8%Ni)
- Main phase is austenite
- Very wide range of applications very low temperature service (cryogenic) high temperature service
- Moderate corrosion resistance
- Non-magnetic
- Low thermal conductivity (‘hold’ the heat during welding)
- High coefficient of expansion – more distortion during welding
4. Duplex stainless steel, as its name implies, is a mixture of two structures or phases, austenite and ferrite. Modern duplex alloys contain nitrogen, which not only adds strength and corrosion resistance, but also improves previous problems with weldability. Likewise, the higher alloyed duplex materials are known as the superduplex alloys. A series of lowered alloyed duplex materials, commonly called “lean duplex”, offers higher strength with corrosion resistance similar to the standard austenitic grades. Where their high strength can be utilized, they can be very cost effective, and potentially have wide application in the water industry.
* Duplex Grades:
- Alloyed with Chromium & some Nickel
- Examples – 22%Cr + 5%Ni & 25%Cr + 7%Ni
- Called duplex because there are 2 phases – 50% ferrite + 50% austenite
- The presence of ferrite means that the steels can be magnetised
- Stronger than 304 & 316 and good resistance to certain types of corrosion
- Not suitable for very low temp. service or very high temp. service
5. Precipitation-hardened materials are alloys whose structure and strength properties can be modified by heat treatment.



- Other way classificate Stainless steel:

Comparison of standardized steels:


- In stainless steel, the chromium is added during the melting of the steel and forms a homogeneous mixture with the iron and other alloying elements, such as nickel and molybdenum, which enhance the metal’s resistance to corrosion.


It will be best if there’s a comparison table between each grade with regarding to welding ability & metallurgy.
LikeLike
we will talk about it later!
LikeLike