Ever wanted to know more about the Galvanic Series? Read on to find out about what it is and how to use it to analyze the compatibility of joining (fastening) metals.
In this article, we’ll look at an example to illustrate the use of the galvanic table.
You can also learn more about overcoming potentially compatibility issues between metals.
What exactly is the Galvanic Series?
The Galvanic Series, also called the electro-potential series, lists metals in the order of their nobility. (Noble metals are those that are resistant to corrosion and oxidation.) When two metals are immersed in an electrolyte, while also being connected externally by a conductor, the less noble metal experiences galvanic corrosion. The rate at which it corrodes depends upon the medium (in this case, the electrolyte) and the difference in nobility of the two metals.
How is the Galvanic Series used?
In general, the farther apart the metals are in the galvanic series, the greater is the corrosion when used together. The galvanic series applies to a specific electrolyte solution – in other words, for each electrolyte that is actually used, a different order or series ensues.
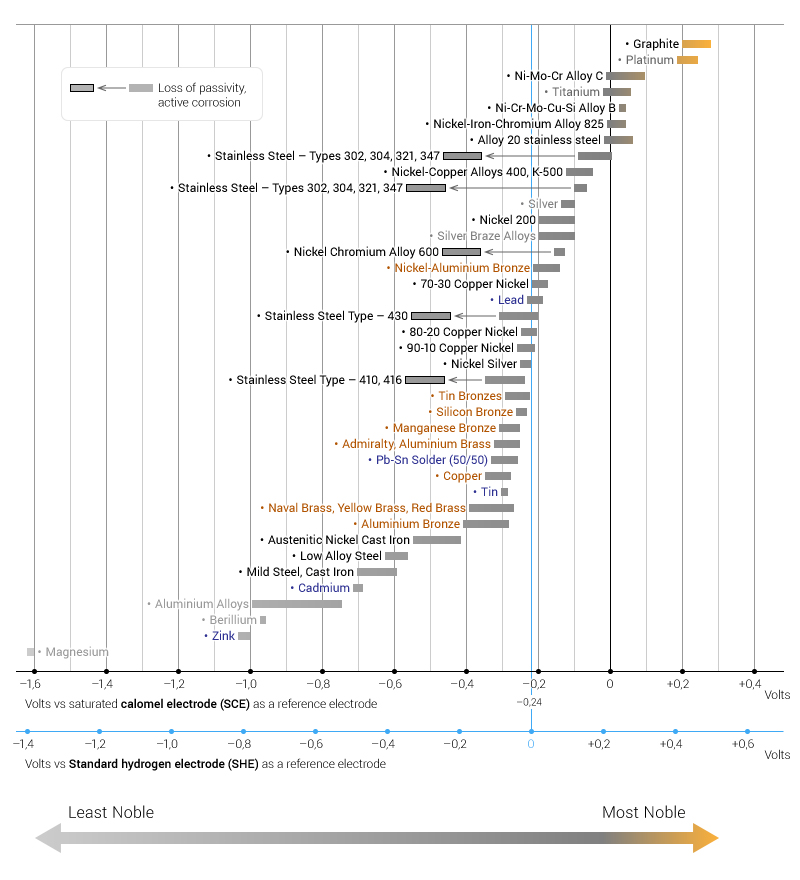
The table below is the galvanic series of metals, alloys and graphite in seawater (most noble at top) in flowing seawater, at ‘normal’ temperature. Maximum recommended voltage difference is 0,2V:
Note that graphite, platinum, gold, silver etc. are the most noble and hence corrode at a lesser rate compared to magnesium, zinc and beryllium at the other end of the series that are more likely to corrode.
The difference in nobility can also be measured by a difference in the electrode potential or voltage potential. In a galvanic couple, the less noble metal has lower electrode potential, and functions as an anode (attracts electrons or anions).
Galvanic Compatibility (closed circuit)
The key to avoiding damaging reactions from different combinations of metals is by using metals that are near to each other in the galvanic series. However, there might be instances during which a design calls for a combination of different metals. In such cases, galvanic compatibility can be achieved by any or a combination of the following methods:
- Plating and Finishes – there are different methods of plating and finishing, which protect the anode against corrosion, while also facilitating contact between dissimilar metals.
- Sacrificial Anode – A coating is applied on the cathode that has a similar potential to the anode, thereby minimizing galvanic corrosion.
- Sealing – The metals are coated with a protective layer of paint to prevent direct contact with the electrolyte.
- Increasing the surface area of the anode – Electron flow (current density) and hence, corrosion rates, are reduced when the surface area of the anode is higher than that of the cathode.

Galvanic compatibility could also be predicted by considering the anodic index (electrochemical voltage developed between the metal and gold) of the metals. A difference of 0.25 V – 0.5 V between the anodic indices of the two metals is acceptable in controlled environments, while the same should ideally not be more than 0.15 V in harsh environments like those with high humidity and salt content (offshore-condition).
Precautions while using the Galvanic Series
- This series does not specify the actual rate of corrosion and hence, should be used only as a qualitative guide while selecting the metals (first-approach).
- A basic knowledge of the environment in which the metals are used, is necessary. Factors such as temperature and humidity vary in different areas. Additionally, a particular metal could have different energy potentials while exposed to air, soil, freshwater or seawater, which in turn affects the rate of galvanic corrosion.
In summary
People today are more willing to experiment with different combinations of metals and alloys as a result of new discoveries in the properties and behaviors of different metals. In such a scenario, the galvanic series acts as a reliable guide to check compatibility of these metals and to predict their shelf life in the environment in which they are used.

