As discussed in earlier post <<What is PREN value for pitting corrosion?>>, pitting corrosion is one of the most common localized corrosion attack and most destructive form of corrosion in metal and alloy.
In article will explain generally about the mechanism and prevention of pitting corrosion:
Pitting Corrosion on Metal Surface
Pitting is one of the most destructive forms of corrosion as it will potential cause equipment failures due to perforation / penetration. pitting generally occurs on metal surfaces protected by oxide film such as Stainless steel, aluminum, etc. Typically for boiler and feed water system, pitting corrosion rate increase dramatically with the increase of oxygen content in the fluid.
Pitting can occur in any metal surfaces. Following are some pictures of pitting corrosion.

Mechanism
Lets look at figure below, oxygen rich fluid in contact with metal surface (at the top of the pit) will becomes the cathode. At the bottom of the pit, low in oxygen level becomes the anode. this will form a complete circuit where metal at the pit (FE) will be ionized to release electron (e) and form ion Ferum (FE2+), this electron will travel to the top of pit to react with Oxygen (O2) (and water, H2O) to form ion hydroxides (OH-). Ion Ferum (FE2+) will react with ion hydroxides (OH-) to form Ferum Oxide (Fe2O3) which typically a brown rust.
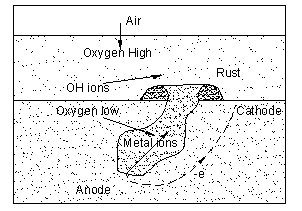
Deeper the pit lesser the oxygen content and higher the potential and pitting corrosion rate.
Severity of pitting corrosion
Knowing that pitting can cause failure due to perforation while the total corrosion, as measured by weight loss might be rather minimal, experience shown that rate of penetration may be 10 to 100 times that by general corrosion, pitting corrosion has been considered to be more dangerous than the uniform corrosion damage because it is very difficult to detect, predict and design against. General metal weight loss method almost impossible to detect the internal pitting corrosion.
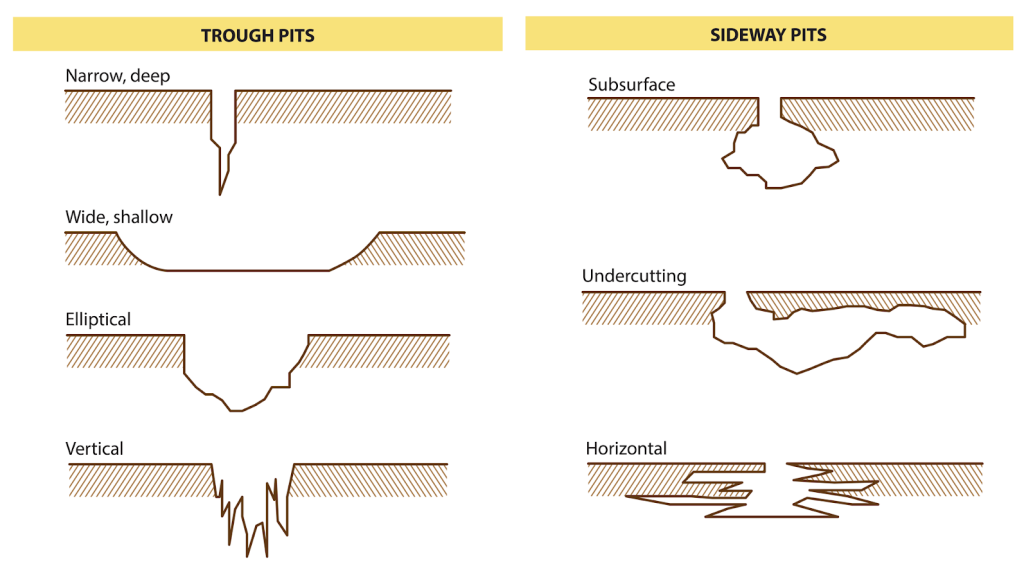
Pitting corrosion shape
Pits formed due to pitting corrosion can become wide and shallow or narrow and deep which can rapidly perforate the wall thickness of a metal. Following picture demonstrate several types of pitting corrosion shape. This has made it even more difficult to be detected especially undercutting, subsurface and horizontal type.
Preventive measures
There are several preventive approach to avoid pitting. There are :
- Proper material selection e.g. SS316 with molybdenum having higher pitting resistance compare to SS304
- Use higher alloys (ASTM G48) for increased resistance to pitting corrosion
- Control oxygen level by injecting oxygen scavenger in boiler water system
- Control pH, chloride concentration and temperature
- Cathodic protection and/or Anodic Protection
- Proper monitoring of oxygen & chloride contents by routine sampling
- Agitation of stagnant fluid
In addition of corrosions in metal, there are many type of corrosion depending on the condition of application. Here are some of them for your reference:


