Stainless Steel material: ( refer to other topic: what is stainless steel? )
A metallic alloy (i.e., metal made of multiple elements such as chromium, nickel, and molybdenum) is generally considered to be “stainless” when its chromium content is greater than about 12 percent by weight ( some document mention it is about 10.5% chromium ), with the balance being iron, higher alloyed stainless steels have higher levels of chromium. Chromium provides corrosion resistance to these alloys by forming a thin, adherent, corrosion-resistant oxide film on a clean (e.g., pickled, wire/rotary brushed, or ground) surface (of the alloy).
The main requirement for stainless steels is that they should be corrosion resistant for a specified application or environment. The selection of a particular “type” and “grade” of stainless steel must initially meet the corrosion resistance requirements. Additional mechanical or physical properties may also need to be considered to achieve the overall service performance requirements.
Passivation is a chemical process that is used to help restore contaminated stainless steel to original corrosion specifications. Parts are placed in baskets and submersed into a diluted nitric acid or citric acid solution for a specified length of time and temperature. This process is designed to remove free iron or other foreign matter from the surface of the metal. Other than giving the part a clean finish, the process does not brighten or change the part appearance.
It has these below steps: cleaning / pickling / passivation and other define – descaling.
Cleaning process:
Prepares the substrate by removing rust, grease, oils and other foreign deposits. The cleaning phase will brighten up the surface of the stainless steel. Degreasing the substrate is essential for proper pickling and uniform formation of the passive layer.

Pickling process:
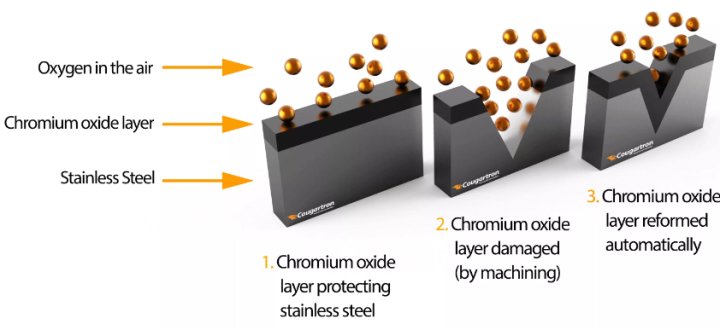
Pickling removes the passive layer in addition to a small amount of metal from the surface of the stainless steel. After pickling, they surface of the stainless will start to oxidize when exposed to oxygen in the air. It is this critical process that restores the corrosion resistance of the stainless steel.

Although this process occurs natually on the surface of stainless steel, it is often useful to accelerate and assist in the development of the passivation layer. Passivation occurs when chromium oxidizes with the oxygen in the air to form a corrosion resistant passivation layer. This process can be accelerated and assisted with a passivation product, ensuring the development of a uniform and thick passive layer.

SUMMARY:
Pickling (Chemical de-scaling) of stainless steel is required to remove scale, heat tint & to create the self-repairing oxide film on surface which enables it to become more passive and corrosion resistant.
Passivation Process:
Many grades of stainless steel can be treated with passivation to remove free iron and contaminants from the surface. However, stainless steel passivation is not recommended for some grades of stainless steel due to inadequate levels of chromimum and nickel. Passivation of stainless steel is also not recommended for parts that have been welded or brazed. Passivated stainless steel doesn’t benefit from removed heat tint or oxide scale as a result of heat treating or welding.

+ THE STAINLESS STEEL PASSIVATION PROCESS
Passivation of metal is a metal finishing operation that many industries specify for their parts. Passivation is a chemical process designed to increase the corrosion resistance of stainless steel parts. To passivate stainless steel parts, the parts are placed in a basket and submerged in a nitric or citric acid bath. The acid concentration, temperature and time the part spends in the bath depends on the stainless steel alloy of the part itself. The process of passivating stainless steel effectively removes free iron and foreign materials from the part’s surface, leaving the part clean and more corrosion resistant. Unlike electropolishing of stainless steel parts that results in a bright and shiny part, passivation of stainless steel parts does not change the parts’ aesthetics.
+ WORKS WHERE PASSIVATED STAINLESS STEEL DOES NOT
Several popular grades of stainless steel cannot be passivated due to low levels of chromium and nickel. When these stainless steels are passivated by standard methods, the resultant flash attack actually weakens the base material and does irreparable damage to the part.
By dissolving surface metal, remove deeply imbedded contamination, reduce surface area and remove the false or amorphous layer that is produced by grinding, machining, stamping or lapping metal. Less imbedded contamination and less retained surface moisture mean less chance for corrosion to begin.
The passive layer of the stainless steel has a chemical composition which is particularly different from the basic composition of the same:
- Around 65%Cr + Chromium oxide
- Around35%Fe + Iron oxide
Molybdenum and Nickel have a very low percentages within the passive layer.
Not always the intervention of the passivation phenomena lead to passive conditions. In the case of stainless steels, the colored oxide films which are observed during the welding phase or black scales that are formed during the hot rolling are less protective of the crome of oxide film that forms on the metal surface.
Normally, the protective oxide layer is often about 1.5-2.5 nm, and is easily visible through special and expensive microscopes (TEM). The term passivation derives from the fact that chromium has a strong affinity with oxygen. When the steel is in contact with an oxygen-rich environment, the chrome is very reactive and tends to form very stable oxides and hydroxides. These compounds are protective because they suppress unwanted reactions that can lead to the stainless steel corrosion. Thus, the stainless steel corrosion resistance derives from the fact that it has a percentage of chromium equal or greater than about 18%. Thus, the stainless steel has the opportunity to spread locally some chromium particles on the surface to form the oxides that enhance the corrosion resistance of stainless steel. The passive layer that forms on the surface of stainless steel is equipped with electronic conductivity then it can generate the chemical oxidation-reduction processes with oxygen that can stop the corrosive circuit.
The percentage of chromium and other substances present in the steel is one of the parameters which influence the quality of the passive layer. A steel of the AISI 200 series will have a lower corrosion resistance compared to an AISI 304 because, having a lower concentration of Nickel, does not have the ability to reform quickly the passive layer after, for example, an abrasion processes and / or pickling.
If the surface finish has been subject to mechanical abrasion (satin finish):
- The microstructure is not homogeneous
- It presents the contaminated abrasive substances that interlock on the surface, becoming points for pitting corrosion
- Reduction of the passive layer thickness.


The construction of the passive layer also depends on the thermodynamic characteristics (temperature, oxidizing environment, etc ….) that allow to adjust the passive layer in order to obtain a stable and durable layer over time.
During standard operation, the quality of the passive layer is independent of:
- Clean air
- Pure water
- Passivation in concentrated nitric acid at 5% -30%
These factors listed above govern the time of passivation of stainless steel:
- Clean air: around 48-96 hours
- Pure water: around 6-15 hours
- Passivation in concentrated nitric acid at 5% -30%: around 30-120 minutes

SUMMARY:
Proper Passivation with a nitric acid-based agent will dissolve surface contamination and assist in the optimal restoration of the chrome-oxide passive layer. If Passivation is not carried out, stainless steel can rust due to surface-free iron and, since chlorides are also often absorbed from the atmosphere, some ferric chloride will be produced. Prolonged contact with ferric chloride will eventually initiate pitting and crevice corrosion on the stainless steel surface. Failure of stainless components due to corrosion can reach catastrophic proportions. The cost in time, materials, rectification, and lost production can be tremendous.
Finally the quality of the passive layer is determined by the percentage of alloying substances inside the steel and the thermodynamic conditions of the environment, promoting a compact and chemically stable layer. The passivation time is determined by the different environments in which the steel is exposed.
Note:
What is DESCALING process?




One thought on “Stainless Steel Material: what is pickling / passivation?”